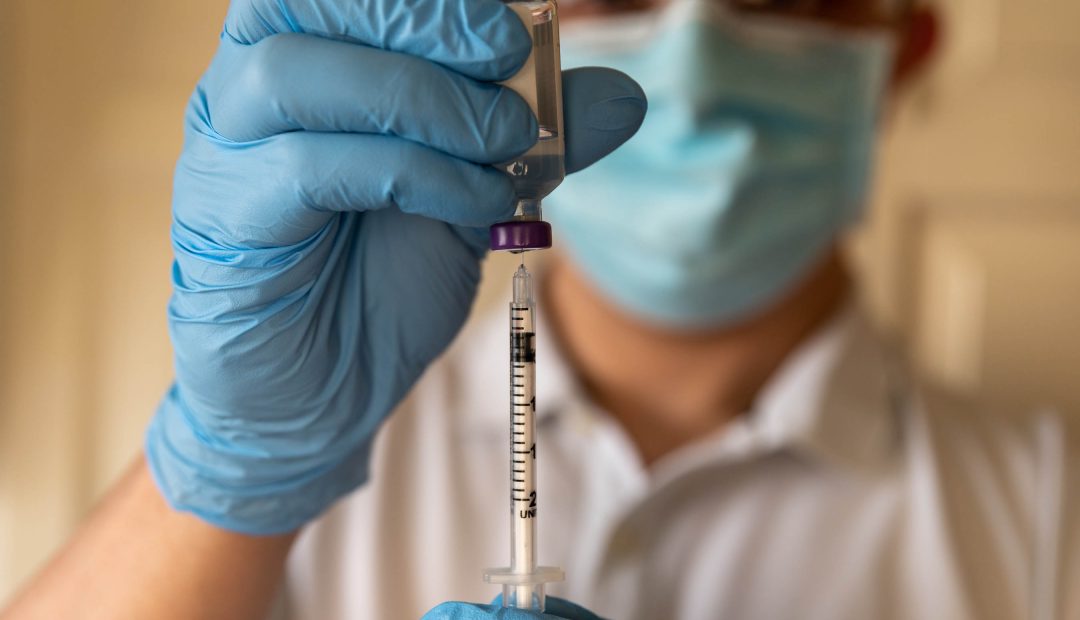
The United States Food and Drug Administration (FDA) has just approved an injectable form of “PrEP.” Currently, PrEP is taken orally and known by brand names Truvada and Descovy, among others.
According to The Washington Post, which cited the CDC, oral PrEP is up to 99% effective when taken daily, but its efficacy drops when intake becomes sporadic or patients begin missing doses.
The new injectable form of PrEP, which goes by the name of Apretude, seeks to solve this problem by becoming a bi-monthly injection. To start, patients will get two shots, one month apart. After that, patients will only need one shot, given every other month.
According to studies, Apretude is much more effective than oral PrEP (largely due to patients not missing doses). In the studies, gay men and trans women had a 69% reduction in infection rates, and cis women had a 90% reduction in infection cases, proving that Apretude could be a game-changer in the fight against HIV/AIDS.
According to NBC News, Dr. Debra Birnkrant, the director of antivirals division at the FDA’s Center for Drug Evaluation, said, “Today’s approval adds an important tool in the effort to end the HIV epidemic by providing the first option to prevent HIV that does not involve taking a daily pill. This injection, given every two months, will be critical to addressing the HIV epidemic in the U.S., including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option.”
Apretude will be shipped and become available to the public in early 2022.